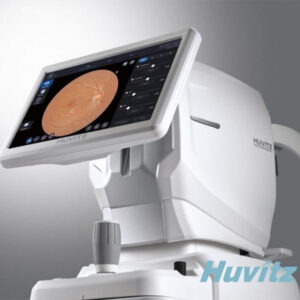
– Expected to play a driving role in entering the U.S. market.
Huvitz, a company specializing in Ophthalmology medical devices (CEO Kim Hyun-Soo), announced on the 4th that its Fundus Camera, ‘HFC-1’, has received medical device certification from the U.S. Food and Drug Administration (FDA).
The North American Ophthalmology diagnostic device market, estimated at approximately 1 trillion KRW in 2021, is the largest Ophthalmology diagnostic device market in the world, where all global top-tier companies compete. It is also a market with the highest entry barriers. Despite the economic downturn caused by COVID-19, the market is expanding at a high annual growth rate of around 6% due to the increased use of bio and advanced medical devices, as well as aging populations and rising life expectancy.
The Fundus Camera ‘HFC-1’, which has recently obtained certification, is a device that illuminates the fundus by directing light onto the pupil and records the fundus condition through the reflected light. It is equipped with Huvitz’s innovative optical algorithm. The device enables the acquisition of high-quality images in any clinical situation, capturing even subtle changes in the retina, allowing for the examination of various fundus-related diseases such as macular degeneration and glaucoma.
The company stated that the North American market is a leading market in the global Ophthalmology medical device industry in terms of technology and trends, and that passing the strict evaluation criteria of the FDA is a recognition of Huvitz’s technological capabilities and quality.
Additionally, with the FDA approval of this fundus camera, the company expects the certification process for many more products to proceed quickly in the future.
In particular, the company mentioned that the support from the Korea Health Industry Development Institute (KHIDI) for the Global Commercialization and Market Expansion of Innovative Medical Devices has made it even more meaningful, as it has established a foundation for the entry of domestic technology into the North American market.
A Huvitz representative stated, “We have been preparing for the FDA certification with the goal of entering the North American market since early last year,” and expressed optimism, saying, “With the certification of the HFC-1, we expect it to play a driving role in not only actively targeting the North American market, including the U.S., but also in spearheading our efforts to expand into the U.S. market.”
Huvitz currently generates approximately 85% of its total revenue from overseas, with the majority of its international sales coming from North America, including the U.S., Europe, and Asia. With this certification, the company expects to expand its export items in the U.S. and diversify its sales channels, leading to increased revenue.
[끝]